Is Using Bromine in Your Pool or Spa with UV or Ozone bad?
Veritasium youtube channel: Why are 96,000,000 Black Balls on This Reservoir
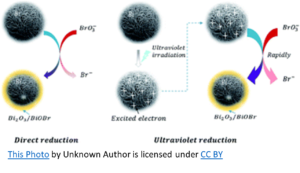
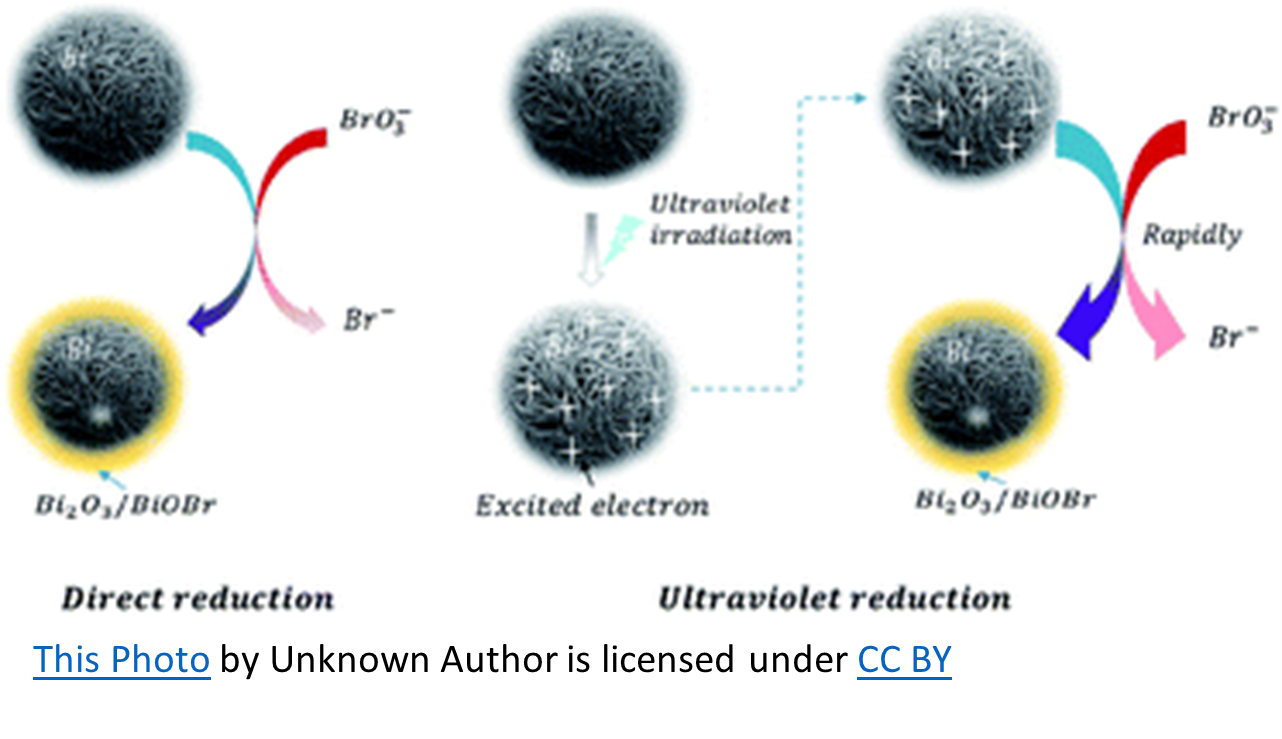
BROMATE: BrO3–
There are many benefits in choosing bromine for use over chlorine for sanitation in an indoor swimming pool. Many aquatics venues go that route. After all, bromine is an effective sanitizer at higher ranges of pH, where chlorine is not. The combined form of bromine, monobromoamine (NH2Br), is an excellent sanitizer, where the combined form of chlorine, monochloramine (NH₂Cl), is fairly weak and ineffective. Monobromoamine is not an irritant and does not have an odor, where monochloramine is foul-smelling (that stereotypical “indoor pool smell”) and can cause discomfort to both the skin and eyes. Bromine is even a better algaecide than chlorine, but there may be a need to light its darker side.
Sounds good so far, so what’s the issue?
When used in conjunction with a supplemental oxidizer such as Ozone or UV, sodium bromide has the potential to become problematic. When water containing bromide ions (Br-) is ozonated, hypobromite ions (OBr-) are formed and become hypobromous acid (HOBr) at the usual aquatic venue pH (7.2 – 7.8). Hypobromous acid, as hypochlorous acid (HOCl), is to Chlorine, is the killing form of Bromine. In the ozonation process, bromate will form due to the oxidation of hypobromite ions by ozone. Bromate is hazardous when ingested and is regulated as a disinfection by-product in drinking water.
Disinfection By-Product: a secondary, often harmful, product that is created as a “side effect” of the synthesis of chemicals
Yeah, but what about UV?
UV may have actually received a bad rap regarding this DBP (Disinfection By-Product) when used with bromine. There is no evidence that UV oxidation alone contributes to bromate (BrO3–). However, in UV/chlorination (or UV/persulfate) oxidation, bromate formation is recognized. Therefore, when used with UV and some other form of oxidation, Sodium Bromide forms some bromate. The question is, does it form enough to pose a threat?
Ozone and UV are both powerful oxidizers and use of either can benefit water quality tremendously. However, caution should be exercised when matching a supplemental oxidizer to a primary disinfectant. When in doubt, consult your local Pool Professional.
Algae Prevention & Eradication Specialist Certification Course: Click Here!
Aren’t Bromine tablets mostly just chlorine anyway?
Bromine tablets are manufactured with a hefty amount of chlorine in the ingredients; the Cl (chloro) in the formula, 1-bromo-3-chloro-5,5-dimethyl hydantoin (C₅H₆BrClN₂O₂), represents this. It is not in there by accident. We put it in there because we need to have it under normal circumstances (minus additional oxidation). Bromide ions, by nature, are lazy. Without some oxidizer added as a kick in the pants, they do not do much of anything. The reaction between chlorine and bromine is actually a displacement reaction (oxidation-reduction); Chlorine displaces bromine from the sodium bromide. So, chlorine oxidation of sodium bromide is built right into the tablet!
Subscribe to Pool Operator Talk News
Okay, but how is that a factor?
To simplify, chlorine oxidizes sodium bromide resulting in free bromine (hypobromous acid and hypobromite ions). The UV splits the free bromine to create hydroxyl radicals that can then react with bromine to form bromate. However, the amount of hydroxyl radicals produced in this manner is minimal, as is the bromate formation. Bromate formation requires an oxygen-based chemical such as ozone, (maybe) persulfate, or hydroxyl radicals. The latter can be formed from UV breakdown of chlorine and to a lesser extent, from UV breakdown of bromine.

So, is Chlorine the problem?
Oxidation of sodium bromide through chlorine alone does not produce bromate, so using bromine tablets with chlorine as an oxidizer, provided that a supplemental system (especially Ozone and to a much smaller extent UV) is not in use, does not constitute a significant threat of BrO3– formation in an indoor application. Chlorine dioxide will also not result in bromate formation, but it will not produce hypobromous acid either.

Can we eliminate the threat?
No, not completely. However, if we wanted to minimize the formation of these carcinogens when UV is in use, we could change up the delivery and use a sodium bromide/bromine tablet combo instead. We can establish a “bromide bank” in the water, which would allow the chlorine (from the tablets) to oxidize sodium bromide ions to bromine quickly, preventing the chlorine from ever reaching the Ultraviolet system. Yes, bromine will still split, but it is much less sensitive to UV than Ozone.

With Ozone in place, the answer is not as simple.
Ozonation of bromine will form bromate without another oxidizer, whether the delivery is bromine tablets or not. Therefore, with ozone, the formation of this DBP is of greater concern. We can take steps to minimize these by-products, but our efforts will likely be futile. Still, we need to consider that BrO3– is not known to be absorbed by the skin and is not volatile. It is taken in through accidental ingestion, and people do not typically drink copious amounts of swimming pool water.
Dammit, Jim! I’m a Pool Guy, not a Doctor… (a nod to the Trekkies)
so, keep in mind; the method of bromate intake described above is as it has been explained to me.

Is there anything we can do?
Studies have shown that increasing/decreasing the pH levels; increasing Total Alkalinity, dissolved organic carbon, or ammonia may be successful in limiting bromate formation. However, the World Health Organization reports that natural organic matter and high carbonate/bicarbonate ions concentrations can mask the actual level. Historically, ion chromatography is the preferred method of testing water for the presence of bromate, though photometric options now exist.
Similar Article: Available Chlorine Content vs. Active Strength… Huh?
https://open.spotify.com/episode/3ZAeQpeh6sLL9MopSvYNVm?si=1b57696bba69454c
References:
Richard Falk with an after-hours chemistry assist
NATIONAL PRIMARY DRINKING WATER REGULATIONS, United States Environmental Protection Agency
Peng-Fei Chao, ROLE OF HYDROXYL RADICALS AND HYPOBROMOUS ACID REACTIONS ON BROMATE FORMATION DURING OZONATION, ARIZONA STATE UNIVERSITY, December 2002
PJ Tynan, DO Lunt, and J Hutchison, THE FORMATION OF BROMATE DURING DRINKING WATER DISINFECTION, WRc plc, Buckinghamshire, UK. 1992/93
Jingyun Fang, Quan Zhao, Chihhao Fan, Chii Shang, Yun Fu, Xiangru Zhang, BROMATE FORMATION FROM THE OXIDATION OF BROMIDE IN THE UV/CHLORINE PROCESS WITH LOW-PRESSURE NAD MEDIUM PRESSURE UV LAMPS, CHEMOSPHERE, 2017
Jing-Yun Fang and Chii Shang, BROMATE FORMATION FROM THE BROMIDE OXIDATION BY THE UV/PERSULFATE PROCESS, Department of Civil and Environmental Engineering, The Hong Kong University of Science and Technology, American Chemical Society 2012
Kishimoto, Naoyuki & Nakamura, Eri. (2012). Bromate Formation Characteristics of UV Irradiation, Hydrogen Peroxide Addition, Ozonation, and Their Combination Processes. International Journal of Photoenergy. 2012. 10.1155/2012/107293.
BROMATE FORMATION DURING OZONATION, Spartan Environmental Technologies
BROMATE IN DRINKING WATER, WHO Guidelines for Drinking-water Quality, World Health Organization, 2005
Rip G. Rice, Ph.D., Chemistries of Ozone for Municipal Pool and Spa Water Treatment, Facts and Fallacies, Rice International Consulting Enterprises






Great article and something I have not read much else about. So is this to say that UV/ozone and chemical oxidizers should not be used in conventional bromine hot tubs to avoid this potential? For hot tub owners using bromine and MPS would using chlorine to activate the bromine be a better option?
Thank you Steve! Yes, both would be better options in an indoor application. In an outdoor body of water, we will still be subject to the same UV rays from the sun that sparked the need for “shade” balls in the LA reservoir as indicated in the video.
Great to know. Thank you for this little science trivia.
You are welcome, Thank you for reading!
This is really interesting! I have never heard of this before – but I don’t have a pool. My grandparents do though!!
I’m glad you enjoyed it. Thank you for reading!
We just switched our condo pool over to bromine. I am so glad they did. It is not as harsh. I will forward this post to our pool guys!
Oh good, I’m glad this can be of help. Thank you for reading!
I learn a lot from your article thank you so much for sharing these amazing information!
Thank you and thank you for reading!
I learn something new everyday!
Thank you for reading!
This is all very interesting. I always thought chlorine and pools went hand in hand with no other options. We don’t have a pool current, but if we get one in the future I will make sure we look deeper into bromine.
There are a few different sanitizers you can use, though chlorine is by far the most popular. Thank you for reading!
Wow i didn’t even know there was an alternative to chlorine. I really dislike the smell as you say so great to have an alternative!
I’m glad you enjoyed it. Thank you for reading! FYI: Chlorine really should not have an odor in water unless it needs to be shocked, but you are correct – bromine does not tend to smell.
Great article! I haven’t heard of this before but it is a rather neat subject and you provided a lot of helpful information. Thanks for writing this.
Thank you and thank you for reading!
This is so so informative! I had no idea about these shade balls. is there a particular type/brand that you use or recommend?
Interesting, right? I’m afraid my Shade Ball knowledge, as far as quality/brand, is minimal. Sorry ? but, Thank you for reading!
I saw a video about the black shade balls on Facebook a while back. I never made the connection that it could be applicable to anything else, so thanks for spreading this information
ikr. The science! Thank you for reading ?
This is something different I read today. I never got a chance to watch black balls video hence this connection is entirely new! Well done.
This is very informative! I loved the way you execute your thoughts in this article. Actually, I have never heard about this before until I read this. Thanks for sharing!
I had no idea this was important. Thanks for sharing.
This is an interesting read! It reminds me of my grade school laboratory lessons.
Very interesting topic and one that raises eyebrows for sure. So are those 96,000,000 black balls making a difference?
a year after these balls were added to the reservoir, the L.A. Department of Water and Power deemed them a success. Unfortunately, a few years later, it was theorized that (from the evaporative loss angle) that it took more water (from other parts of the world) to manufacture the shade balls than they could possibly save.
Wow…there is so much science behind this!
Some ozone manufacturers used to tout the use of bromine with ozone. This is an excellent explanation on the reasons why that is a bad idea. It is also why I strongly discourage pool techs who service my systems from using sodium bromide for algae control. Ozone with a little dose of chlorine is all you should ever need.
Thanks for all this info Rudy. Quick question: Canada has now banned the use of sodium bromide in Spas but not the use of bromine tablets. Are you aware of a difference between the two that would explain why sodium bromide creates a potential cancer risk when used with an ozonator but tablets do not ?
It really depends on the type of bromine tablet used. Bromate (the carcinogen) is formed by the oxidation of hypobromite ions by ozone or UV. 1-bromo-3-chloro-5,5-dimethylhydantoin (C₅H₆BrClN₂O₂), which is the more popular formula will give us both hypobromous acid and hypobromite ions and should be avoided when UV or Ozone is in use. There are tablets with the formula 1,3-dibromo-5,5-dimethylhydantoin tablets and these produce hypobromous acid but do not produce hypobromite ions. With that said, the safest option would be to avoid the combo all together.
Pingback: The AOP Hydroxyl Radical •OH - Pool Operator Talk News🗞
I fotgot to wrote what my brominetablets contains. It contains Bromchlor-5,5-dimethylimidazolidin-2,4-dion.
What could these tablets produce?
Booth Hypobromous acid and hypobromite ions? Or just one of those?
Hi!
I have an outdoor spa with a cover on. The spa has an ozonator and i am using a tablett feeder with brominetablets. After the bath i shock with active oxygen. But after reading this is seems to be a bad idea. What do u recommend if i want to use bromine? Should i turn of the ozonator and not use active oxygen?
You could easily go with a low level of chlorine instead of bromine and eliminate the concern. If it is the bromine tablets you are using now you will not want to use the same feeder for chlorine – speak with your local pool professional about your options.
Thanks for the answer. I have used spachlorine before but got high cya very fast. Thats why I switched. Next time I ewant to change back to chlorine again. But maybe this time chlorine that dont raise cya so fast. Do u have an idea? Calcium hypochlorite maybe? If I use chlorine to a 20-30 ppm in cya and the switch to calcium hypochlorite?