
This may very well be the ‘New Craze’ in swimming pool chemistry and probably comes as close to being the elusive ‘Magic Pill’ as anything. Though not exactly ‘New’ – the use of borates in water treatment has been a ‘thing’ for a really long time.
[responsivevoice_button voice=”UK English Male” buttontext=”Listen to Post”]
The team at Pool Chlor had conducted much of the original research using a borate buffering system in swimming pools starting back in the 70s/80s. In the past couple of decades, troublefreepool.com has increased awareness of the many benefits of the boron-oxygen compound.
A few other articles since mostly mirroring the info shared by the above did help a smidgeon keep borates in the front of pool folk minds.

Borates in Swimming Pools – the Stuff Everyone Talks About:
• Improve swimmer comfort—reducing red-eye and skin irritation.
• Soften water.
• Provide good algal control.
• Reduce scaling.
• Improve water clarity.
• Reduces corrosion.
• Save energy.
• Be added easily and dissolve instantly.
• Improve oxidizer performance and longevity (typically chlorine).
• Provide exceptional buffering capacity.
Similar Article Lowering Total Alkalinity Without Affecting pH
Buffering is The Main Reason We Use Borates in Swimming Pools.
Establishing a buffering system in a swimming pool makes it so the water can resist a pH change. We do this by establishing a level of a weak acid (like carbonic acid or boric acid) and its conjugate base (like bicarbonate or tetraborate).
This solution in the water can neutralize strong acids or bases by turning them into something else. The buffer makes that happen by donating or accepting a proton (hydrogen ion) when the pH level is threatened.
.
You’re probably more familiar with these terms when talking about Total Alkalinity. In fact, in most certification classes for pool operators, we give the Total Alkalinity (TA) all of the credit.
We say that TA is the water’s ability to resist a change in pH. Total Alkalinity can do this because bicarbonate, along with carbonic acid, is a bicarbonate buffering system. This buffering system will buffer against the pH moving up or down but does its best to prevent it from going down.
Buffer in Both Directions.
So if we already have Total Alkalinity, do we need to have borates as well? Ideally, YES! Even though a bicarbonate buffering system (TA) and a borate buffering system will both work to prevent a change in pH, they tend to specialize in preventing a pH drift in one direction over the other at the pH range we typically keep in swimming pools. It all comes down to the pKa value.
pKa Value?
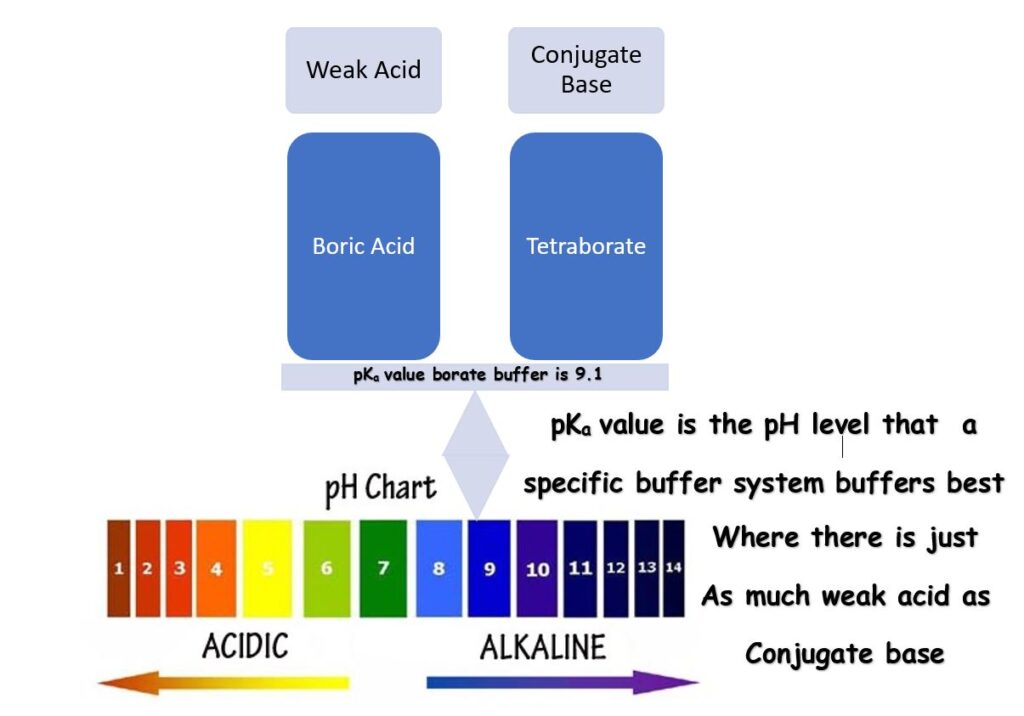
A pKa value is the perfect pH! Not talkin ’bout your ‘ideal range’ for water testing here, but the pH at which a specific buffer system buffers best. Some refer to a pKa value as a means to measure the strength of an acid, which it is, but we’ll save that for another conversation.
When we add a weak acid to the pool water, an amount of conjugate base, which is also weak, forms, for example, we know that many large commercial facilities use CO₂ (Carbon dioxide) for pH control.
This works because upon injection, the CO₂ becomes carbonic acid (weak acid). We are also aware that this is the only means of lowering pH that will increase Total Alkalinity.
This occurs because carbonic acid (H₂CO₃) donates a hydrogen ion becoming hydrogen (H⁺) and bicarbonate ions (HCO₃⁻). Bicarbonate ions are the conjugate base of carbonic acid (weak acid). We also know that pH affects the percentages of things, and this is where our ‘Perfect pH,’ our pKa value comes into play.
Hydrogen ion Give & Take.
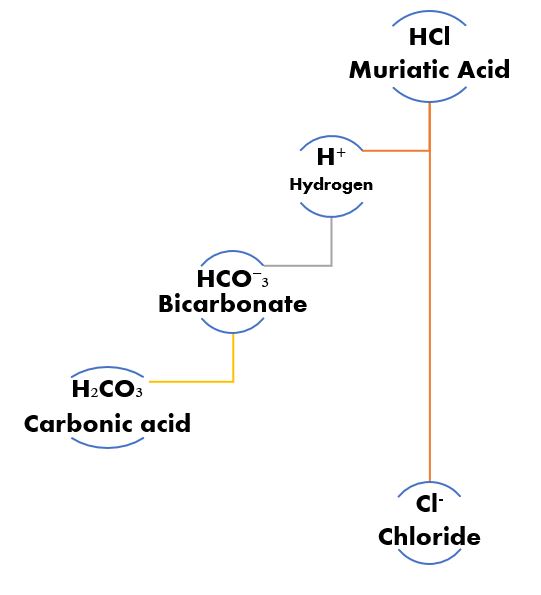
Let’s say muriatic acid (HCl) is added to water where we have established a bicarbonate buffer system. The bicarbonate ion base (HCO⁻₃) accepts a proton (H+) becoming Carbonic acid (H₂CO₃), which is simply HCO⁻₃ (with only one H+) + the H+ from HCl going to H₂CO₃ (a quantity of two H+). The HCl losing an H+ becomes Cl– which is simply chloride. By changing the acid to something else the effect of the dose of acid on pH is changed.
Follow Us on FaceBook HERE
The pKa value for a specific buffering system is the pH where you have just as much of your weak acid as you have of your conjugate base, a 1: 1 ratio. For your bicarbonate (Total Alkalinity) buffering system, that pH is 6.1, so 6.1 is your pKa value.
Any pH reading above 6.1 would indicate that we have more bicarbonate ions than carbonic acid. Anything below a pH of 6.1 would mean we have more carbonic acid than bicarbonate.
The pKa value is the pH where a specific type of buffer buffers best
This one to one ratio we have at a pH of 6.1 makes 6.1 the pH where our bicarbonate buffering system buffers best – where we have our greatest buffering capacity.
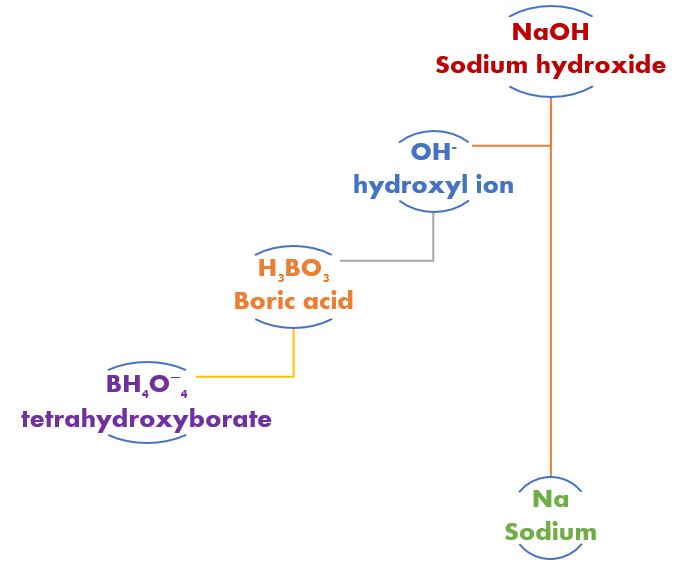
With our borate buffer system in place, should we add a dose of sodium hydroxide (caustic soda, lye). The boric acid would neutralize that dose but not in the traditional give a proton kind of way. In fact, boric acid is not very giving acid at all. It’s more of a take, take, and type of thing, and it isn’t even a measly old proton. Nope, it wants more.
Boric acid (H3BO3) neutralizes a strong base like NaOH (sodium hydroxide) by taking a hydroxyl ion (OH-) and becoming tetrahydroxyborate (BH4O−4). By changing the acid to something else, the dose of acid’s effect on pH is changed. By changing the base and itself to something else, the dose of a strong base on the water’s pH is changed.
.
A borate buffering system works the same but totally different. Boric acid does not donate a hydrogen ion as we had seen with carbonic acid. Instead, boric acid (H3BO3) takes a hydroxyl ion (OH–) from the water to form its conjugate base, tetrahydroxyborate (BH4O−4).
We also see the possibility of several other borate species existing within those same ranges as a conjugate base. That is because boric acid breaks the buffer rules by taking instead of giving in its not donating a proton.
What about Borax?
Borax (disodium tetraborate decahydrate) can also be used if activated by an acid such as hydrochloric acid, which creates a Borax/HCl buffer system.
Regardless, with a borate buffer system, 9.1 is our perfect pH, and that, of course, is our pKa value for that system. Like the bicarbonate buffer system above, a pH of 9.1 is where we have equal amounts of boric acid and tetrahydroxyborate (or whatever conjugate base was actually formed) and where our borate buffering system buffers best. At any pH of less than 9.1, we will see more boric acid.
One up, one down!
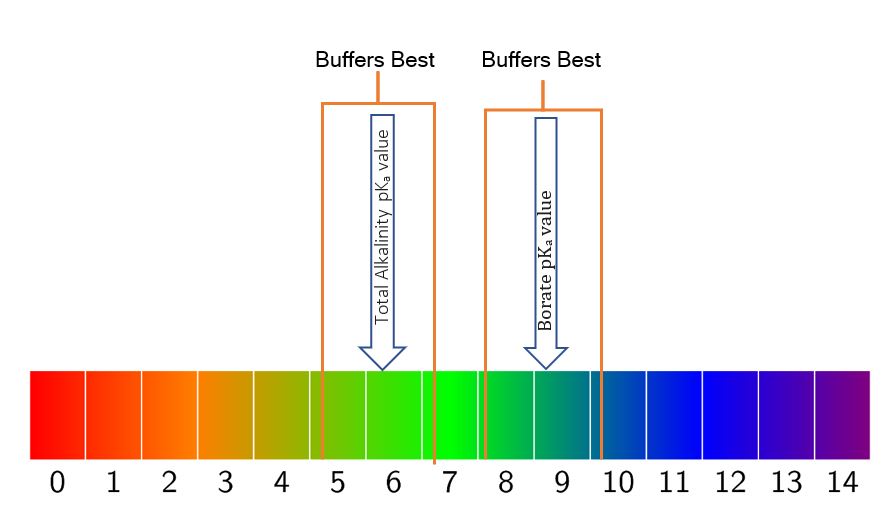
The cool thing is that we get a little bit of leeway, and that is exactly what makes these two buffering systems (bicarbonate & borate) an ideal combo for pool use. One whole number up on the pH scale and one whole number down is what we get for ideal buffering capacity.
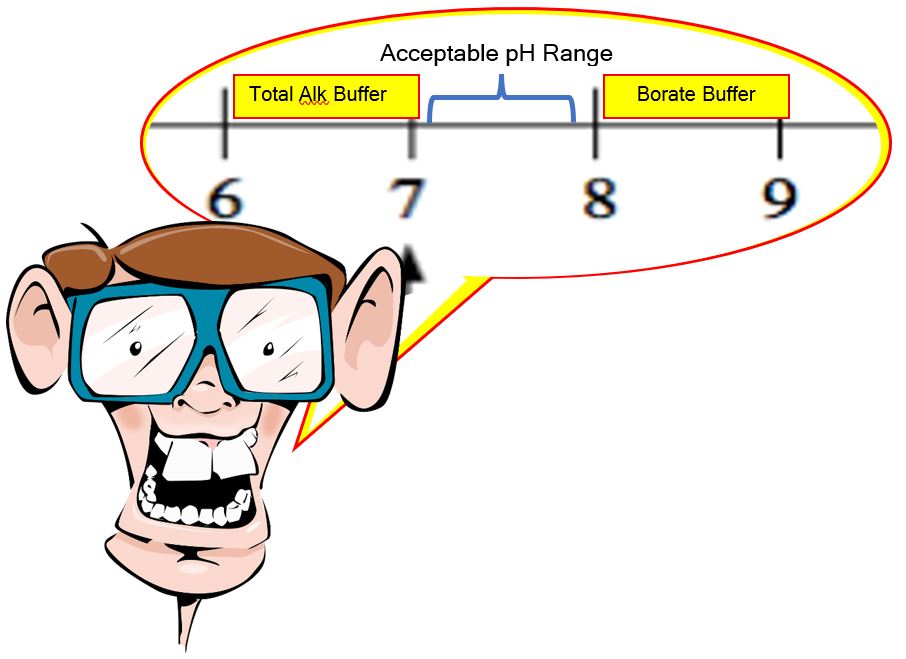
That gives us a range of 5.1 to 7.1 on a bicarbonate buffering (Total Alkalinity) system and then from 8.1 to 10.1 for our borate buffer.
Borates in Swimming Pools, This Photo by Unknown Author is licensed under CC BY-SA
If you take the high on the TA range and the low from the borate range, that bookends our acceptable range of 7.2 to 7.8 almost perfectly. You can pretty much keep your pH ‘locked’ in place by maintaining a Total Alkalinity of 80 to 100 ppm, a Cyanuric acid (yes, this has a pKa value and buffers a bit itself) of 30 to 50 ppm, and a borate level of 50 ppm.
I say ‘locked’ in place with quotation marks because we can’t really lock the pH in place. However, we can make it a lot more difficult to move, and that’s what we do when we establish these buffer systems.
Strength in Numbers
The closer the pH gets to the pKa value (the perfect pH), the greater the level of resistance the pH will meet.
With a borate buffer system in place, the closer the pH comes to a pH level of 8.1 (our one up, one down pKa range), the amount of base to make an upward change increases exponentially.
It would be the same if we recognized a downward drift with an appropriate level of Total Alkalinity. The closer the pH gets to 7.1, the more resistance against change it will face.





Congratulations.
For sure this is a complex well written text.
Outside the USA Borax Is a banned substance????
So for us – no go.
Pratically therefore the solution is simply LSI balance.
Also very interesting is the constant promotion of HCL. However its so corrosive. It (HCL) destroys dispensing systems and the whole plant room. So sulphuric acid doesn’t destroy dispensers, or destroy the plant room and – controls pH so much better.
Side effects – none!
In Europe HCL is the “dirty – cheap – floor cleaner”.
Interesting because I understand and have tried hard to make HCL work, simply sulphuric is sooo much better, more efficient and zero side effects….
That’s interesting, Simon. Do you know why Borax is banned in Spain?
Pingback: Muriatic Acid Alternatives - Pool Operator Talk News🗞
Pingback: Pool Acid Scarcity? Try a Borate-Bicarbonate Buffer - Pool Operator Talk News🗞