The Future of Swimming Pool Disinfection
Advanced oxidation process is defined as those which involve the generation of hydroxyl radicals in sufficient quantity to affect water purification. As a result of the process, we get the AOP Hydroxyl Radical •OH. We say ‘Advanced’ because the oxidation reactions are significantly faster than they would naturally occur otherwise.
[responsivevoice_button voice=”UK English Male” buttontext=”Listen to Post”]

Obviously, this is not a new thing in water purification with references made as far back as 1897; however, it has taken its time in finding its way into mainstream swimming pool water treatment. Realistically we are only just now on the cusp of having our foot in the door, and with its popularity on the horizon, familiarizing ourselves with the basics of AOP wouldn’t hurt.
Advanced oxidation process
Luckily, with the introduction of advanced oxidation processes (AOPs) in drinking water treatment nearly forty years ago, most of the heavy lifting has been done for us. In fact, these methods have been put to the test against some of the toughest of the tough and have made light work of heavyweights such as cyanobacteria (black algae), cryptosporidium, white water mold (WWM), and giardia.
In one manner or another, the hydroxyl radical is the result of the dissociation of hydrogen peroxide (H2O2). The product is •OH, which is the neutral form of hydroxide (OH-). Although short-lived, the hydroxyl radical decomposes organics and inorganics by breaking apart (cracking) the bonds that hold these molecules together.
The AOP Hydroxyl Radical •OH
I know you already know about the superior oxidizing power of UV and Ozone (O3) in swimming pool water. Think of AOP as a method that boosts the level of oxidation offered by each (see Popeye analogy below). This can be done by simply adding a dose of hydrogen peroxide (H2O2) to the mix or by using a combination of both UV and Ozone which besides generating •OH, will create the peroxide we need. Oddly enough, peroxide on its own is not super effective at all.
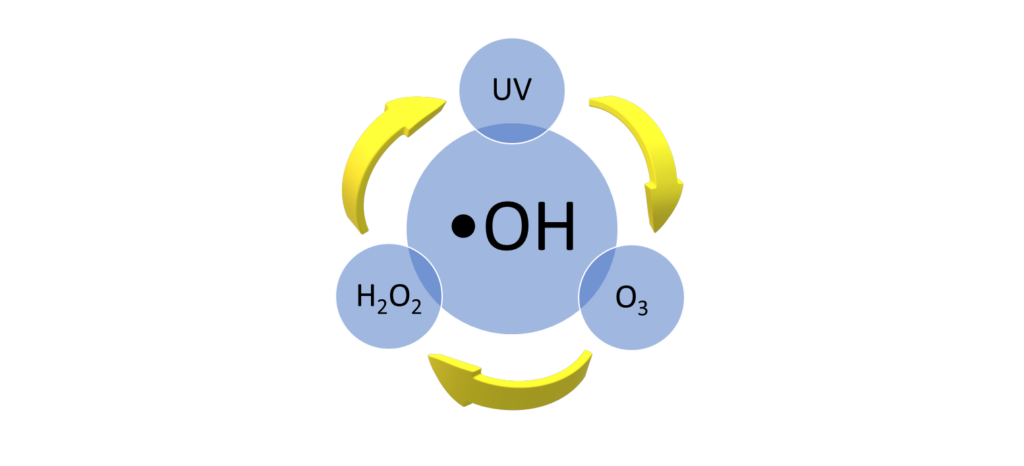
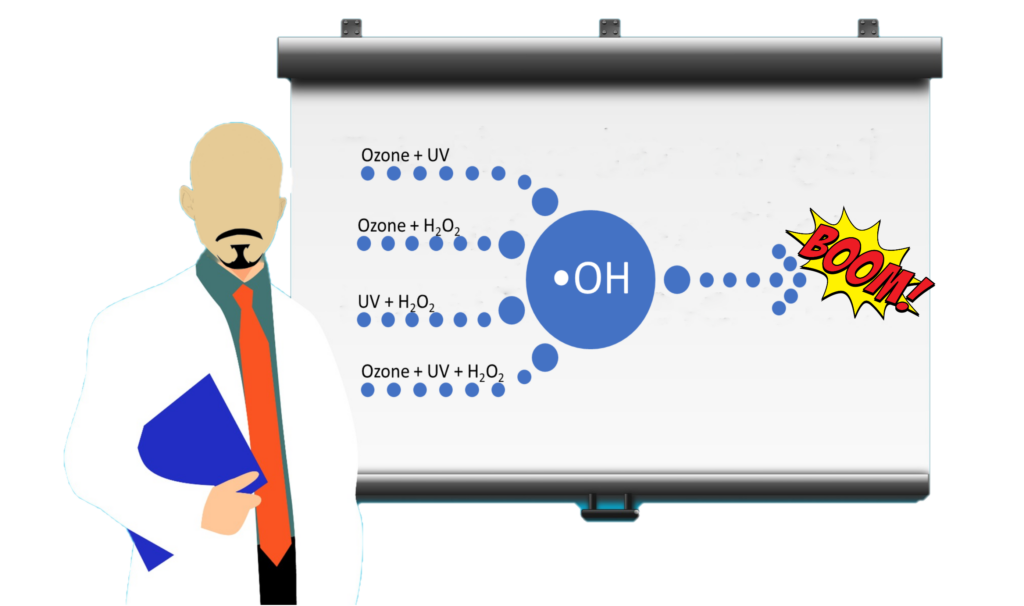
There are a few different methods of generating the hydroxyl radical •OH that we can utilize.
- Ozone/UV.
- Ozone/Hydrogen Peroxide.
- UV/Hydrogen Peroxide.
- Hydrogen Peroxide/Ozone/UV.
Ozone/UV.
O3+ H2O → O2+ H2O2
2 O3+ H2O2→ 2 •OH + 3 O2
UV has enough energy to begin the decomposition of Ozone (UV photolysis), which, when in water which results in hydroxyl radical formation and the formation of hydrogen peroxide. Moreover, the O3/UV method is by far the best means of generating hydroxyl radicals but struggles to produce large quantities due to the low solubility of O3 in water. However, this method basically fell from grace due to cost in comparison to ‘Peroxone’ or UV/H2O2. But, with recent advances in technology, we may again see the Ozone/UV systems also take their place back on the playing field.

Warning: Use of AOP, or Ozone or UV in a pool treated with Bromine, or Chlorine treated pools with Sodium Bromide added as an algae treatment is not recommended. This method will result in the production of the carcinogen BROMATE (BrO3–): Read More
Ozone/Hydrogen Peroxide
H2O2 + H2O → HO2– + H3O+
O3 + HO2– → •OH + 3O2
This is the iron horse of hydroxyl radical (•OH) generation in advanced oxidation. Sometimes called ‘Peroxone,’ the H2O2 injection/O3 method is the most popular and most widely accepted by regulatory agencies. Extensive field testing has provided a proven track record. Consequently, the peroxide/ozone method is much more cost-effective in hydroxyl radical (•OH) generation than the less popular O3/UV systems.
UV/Hydrogen Peroxide
H2O2→ 2 •OH
UV light dissociation of H2O2 is probably the most used method of hydroxyl radical generation at the moment. This is due primarily to UV (ultraviolet light) growing popularity in remediation fecal-related waterborne protozoa such as cryptosporidium and giardia. A huge benefit is the ability of the H2O2/UV process to generate large amounts of hydroxyl radicals. On the other hand, this requires a heck of a lot more hydrogen peroxide than the ‘peroxone’ process described above.
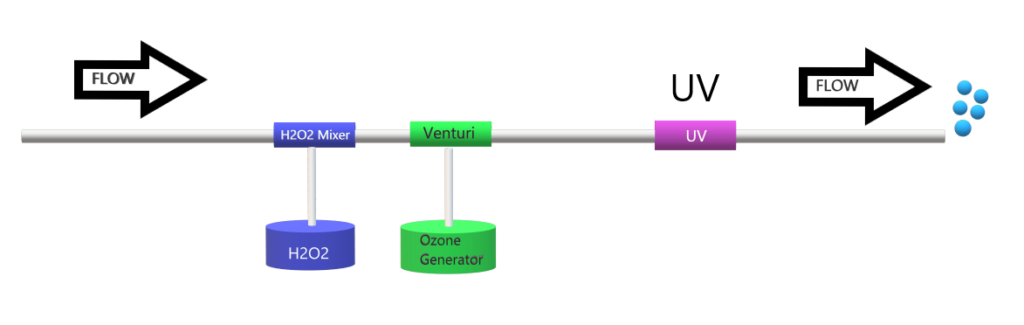
Hydrogen Peroxide/Ozone/UV
OH + H2O2 → •O2– + H2O + H+
•O2– + O3 → •O3- + O2
•O3– + H+ → HO3
HO3• → •OH + O2
We already know Ozone/UV systems that the UV photolysis of ozone leads to the formation of H2O2 and •OH. By adding additional peroxide, we can augment our hydroxyl radical generation. However, the process is cost-prohibitive in comparison to both H2O2/ozone and H2O2/UV.
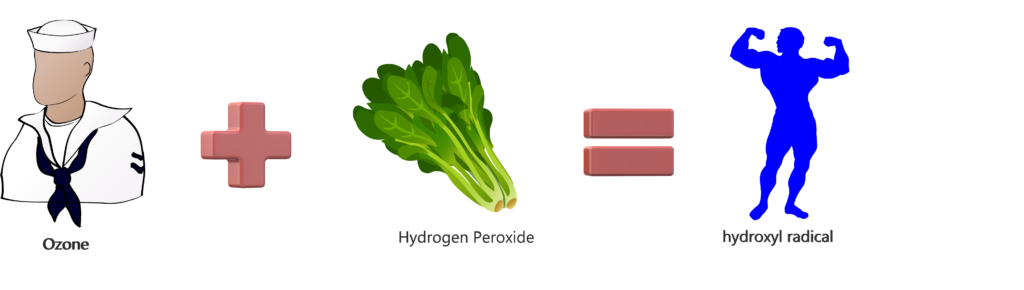
The Popeye Analogy
This is about the easiest way we can explain this to a customer. We all know that Popeye the Sailor was a total badass, right? And, we know his strength really intensified after eating spinach. On the other hand, spinach isn’t all that powerful on its own. So, if Popeye is Ozone (or UV) and Spinach is Hydrogen Peroxide, then Popeye + Spinach = hydroxyl radical •OH
Do you agree that AOP will be the swimming pool water treatment of the future?







Is it administered as a constant source or added periodically like a shock?
Hey Jules! Great question. •OH is extremely short-lived, so when we factor in the Gage and Bidwell Law of Dilution, showing that we’ll require better than four turnovers before >98% of the pool water is treated, this really puts us in a position where continuous operation is needed. When talking about the addition of H2O2 in the process (outside of UV/O3), this is often not metered efficiently and results in much wasted due to self-decomposition.