Copper Sulfate Pentahydrate

Specifics:
“L” shaped Class A Competition Pool; 230K gallons of water; location: Florida; >14 years of documented Black Algae growth; qty of 2 of 7.5 hp Baldor pump; field built vacuum DE – qty 108 of 19” circular grids – 423.36 sq ft of filter area; 675 gpm flow rate – 5.679 hour turn over; uses sodium hypochlorite 10% to 10.5%; Stenner #45M2 set at max 0.42 gph. Water quality visual: Limpid.
Recent FLDOH Inspection Violations:
- Appearance/Algae/Clarity. 64E-9.004(1)(c) & (3); 64E-9.017(1)(c). The pool shall be free from floating debris, sediment, dirt, algae. The main drain shall be visible
- Free Chlorine/Bromine. 64E-9.004(1); 64E-9.017(1). Free chlorine level must be between 1-10mg/L (parts per million) in conventional swimming pools, inclusive; or 1½- 10 ppm bromine. Spa pools & IWFs must maintain 2-10 mg/L free chlorine, or 3-10mg/L bromine. The maximum disinfectant level for indoor conventional swimming pools is 5 mg/L chlorine or 6 mg/L bromine. Pool owner must prohibit pool use when water quality is outside these parameters.
- pH. 64E-9.004(1); 64E-9.017(1). The pH in all pools shall be maintained between 7.2 and 7.8, inclusive. Pool owner must prohibit pool use when water quality is outside these parameters.
- Flowmeter. 64E-9.008(10)(b); FBC 454.1.6.5.13. All pools shall have a functional flowmeter capable of reading from 1/2 to 1&1/2 the design flow rate.
- Equipment Room Drainage/Vent/Lighting/Clean. 64E-9.008(10) & (13);FBC454.1.5.1-.9. Equipment room shall: have Drainage, ventilation, lighting and be clutter free
The method of using strictly copper sulfate is not necessarily the process we would utilize in the treatment of “Black Algae” in a swimming pool. With significant colonization on the floor of a plaster pool, adding a large dose of a slow dissolving granule chlorine (calcium hypochlorite or Trichlor) directly over the areas would typically be our initial approach. This would then leave only those areas on the walls and step sections. If in small amounts, chalking (using a pole attachment that allows a user to rub a chlorine tablet directly upon it). For the test pool in question, the massive colonies that occupy the walls would prevent chalking from ever being an option.
Remember, the purpose of this experiment is to measure the effect of a lower than lethal dose of copper over an increased contact time.
Copper is copper, right? Well, for the most part and most available to us that can be used as an algaecide in a swimming pool (meeting standard NSF60) are chelated to avoid the adverse effects of heavy metal in water (i.e.: staining, etc.). As luck would have it, colleague Doug McKenzie (Earth Science Laboratories) had reached out to talk about my article “the Black Algae myth” at about the time we had identified and secured a swimming pool with colonization for testing. During our discussion on my planned research, Doug offered to supply us with an amount of Earth Science Laboratories AlgaeShield® (copper sulfate pentahydrate) algaecide in a quantity sufficient to treat our 230K gallon test pool. We happily accepted his offer.
 The product arrived within three days. We would need to coordinate our efforts with both the facility and the recently contracted swimming pool service company (about 2 weeks at this point) who continue to maintain the water balance and maintenance throughout our study. In order to verify that the product had been tested and did indeed meet NSF 60, we did contact NSF International directly. The FL DOH requires all chemicals used for swimming pool care and maintenance to meet the NSF 60 standard. Doug had explained that the product manufactured by Earth Science Laboratories boasts a different delivery system. From the information available on their website, it appears (though it is not said) that a spreading agent, similar to the use of paraffin hydrocarbons in the delivery of fatty alcohols used in liquid solar covers, is used. The use of a spreading agent is purely speculation
The product arrived within three days. We would need to coordinate our efforts with both the facility and the recently contracted swimming pool service company (about 2 weeks at this point) who continue to maintain the water balance and maintenance throughout our study. In order to verify that the product had been tested and did indeed meet NSF 60, we did contact NSF International directly. The FL DOH requires all chemicals used for swimming pool care and maintenance to meet the NSF 60 standard. Doug had explained that the product manufactured by Earth Science Laboratories boasts a different delivery system. From the information available on their website, it appears (though it is not said) that a spreading agent, similar to the use of paraffin hydrocarbons in the delivery of fatty alcohols used in liquid solar covers, is used. The use of a spreading agent is purely speculation
Upon receipt, we immediately noted the lack of instruction on the label. The directions simply stating, “Where visible algae are present apply at a rate of 2 fluid ounces of AlgaeShield® per 1,000 gallons of water. This will yield a rate of 0.9 ppm metallic copper. We found the information on addition and maintenance to be less then sparse. Listed are no water chemistry requirements, no instruction on how to add (dilute or not dilute), or any mention of the need to brush; just the dose and the dose alone.
08:00 hrs August 5, 2018:
Water Test Results:
FAC3.0 ppm
TAC: 3.0 ppm
pH: 7.7
TA: 90 ppm
CH: 190 ppm
CYA: 0 ppm
Copper: 0 ppm
Water Temp: 86 Deg F
TDS: 1,750 ppm
LSI: -0.01
Without diluting, we added the recommended dose of the AlgaeShield® product (14.375 quarts) to the swimming pool concentrating the majority of the algaecide into the diving well of the swimming pool. We immediately began the process of brushing the walls and floor of the class A pool with an 18” stainless steel bristle brush. I became immediately aware that it has been a period of time since ⏱ I had personally brushed a commercial swimming pool. ?
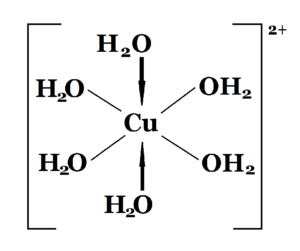
A wave of blue washed quickly throughout the pool, much faster than I have witnessed in other copper sulfate tests I have conducted (evidence of a spreading agent?). By the time the brushing of the competition pool was complete, the water in the pool had developed a turbid blue haze. Somewhat translucent in the shallow end, but visibility levels were less than allowable in the diving well; the main drain grates were no longer visible. This was well beyond the normal hexaaquacopper(II) ion [Cu(H2O)6]2+ “bluing” experiences (regarding visibility) that we were anticipating. The impared visibility most likely due to interaction with carbonate ions forming insoluble cupric carbonate (CuCO3). To remedy, we would adjust both the pH and TA downward. We would need to make the adjustment slowly in order to prevent the chloride ions (from the hydrochloric acid) from displacing the H2O molecules in the hexaaquacopper(II) ion causing the water to turn green (four chloride ions replacing the six water molecules creating tetrachlorocuprate(II) ions); [CuCl4]2-. The green color would be only temporary, but would be much more alarming to the facility.
12:00 hrs August 5, 2018:
After four hours of filtration, the main drain grates were again visible and the polyester filter elements had become a brilliant shade of “smurf” blue. The water was far from the level of clarity the pool had owned prior to the addition of the AlgaeShield®, however had reached a turbidity level that would not delay an opening. The copper level now tested at 0.8 ppm. It would take several days of continual gains in clarity before we would again reach the translucent blue of hexaaquacopper (II) ions, it would be an additional week before the blue would fade to the original crystalline level of clarity. The cupric carbonate (CuCO3) reaction we faced upon addition was likely due to the higher (still acceptable) level of pH & TA we recognized as well as the quantity of product added, however I suspect that the “spreading” agent we had spoken of earlier may have also been a factor.
August 6, 2018: Patrons of the facility take note of change in color and clarity and begin to question the safety of the water.
We found the copper level had dropped extremely quickly and then leveled at 0.4 ppm for the first week following addition. The coper level dropped to 0.20 during the second week. We were aware that a second dose would be required; however, we would need permission from the facility and the existing swimming pool service company before we chance “Bluing” or clouding the water blue once again. We received the go ahead on day 15 and immediately added the maintenance does of 1 ounce per thousand gallons (7.1875 quarts). We did not repeat the initial does of 2 ounces per thousand gallons as we now had an established copper level. A slight, but clear bluing was observed. The color change associated with the second dose was extremely short-lived, lasting the greater part of a day.
We know that trace amounts copper are necessary for photosynthesis and that the process has a fairly high demand. Cyanobacteria must continually uptake metals to support this process. It is also necessary for the prokaryote regulate intake carefully so that intracellular levels do not reach toxic levels. The immediate drop in the level of copper added in the initial dose is presumed to be due to the rapid absorbtion of the copper by the massive colonization present in the test pool. This was not anticipated to be as significant. It is important to note that no water was replaced during this time by backwash or otherwise, the test pool is not equipt with a water leveling device, and that a substantial leak at this facility is not suspected.

Staff continues to brush walls and floors of pool as directed (despite the lack of label instruction) a minimum of one time daily. Cyanobacteria utilizes exopolysaccharidic secretions for “protection” and for the best chance of eradication, the mosaic protein “sheath” would need to be compromised.
The first seven days were uneventful, recognizing minimal improvement if any at all. Heading into the second week, the staff at the facility began to note success. However, I cannot honestly say that I could see any visual improvement myself. Going into the third week following the addition of the copper sulfate pentahydrate, progress would become more obvious. The algae growth had begun to diminish all about the pool.
Also witnessed during week three of treatment was a fair amount of movement with portions of the colonies dispersing through clumping. “Clumping” is one of the noted methods of movement of waterborne biofilm bacteria, collectively dispersing by rippling or rolling across the surface, or by detaching in clumps. They are also able to move as individual cells through a process known as “swarming and seeding dispersal”.
Though visual improvement was evident, it would not provide a sufficient means of gauging success. Through a series of photos we had taken of a single area within the pool, we were able to assemble a time-lapse video of the research:
Still, this was utilizing a visual determination that would not provide a precise measure of success. We reached out to a graphic design firm for assistance. Here we would have the designer create an image histogram, which would plot the number of pixels in a photo of each shade of color. Utilizing the pixel count, we could then determine the percentage of cyanobacteria in the photo to the photo as a whole. Below are the image results supplied by the graphic designer of the Day 1 & Day 28 images as shown in time-lapse video above.
Variances in shading (plus big ass shadow in Day 28) would create an estimated 2% margin for error…

Histogram Results indicate a 50.7414% reduction in cyanobacteria in 28 days of treatment. This is consistent with visual determination results of the pool as a whole.
The copper sulfate pentahydrate as a stand-alone product was a success in greatly reducing the thickness and size of the cyanobacteria colonies present in our test pool. The product did not remove 100% of the growth, however the results are promising and it is anticipated that continued treatment would further reduce the size of the colonies (On the copper’s behalf, the pool did have > 14 years of confirmed “Black Algae” growth)
The research experiment did prove that a dose < 20% the confirmed lethal dose (5.0 ppm copper) would be effective with an increased contact time. Indicating that the cyanobacteria is not immune to copper at less than lethal doses and with what is generally believed a level which would cause stagnation at best may prove sufficient to eradicate.
Did the results warrant the big blue hazy cloud of hexaaquacopper(II) ions to a less desirable cupric carbonate? Had the pH and Total Alkalinity been lowered to the lower end of the acceptable range prior to addition, the “Blue Cloud” would most likely have not occured. I think with a heads up on the label, nobody is going to give a ? about the cloud if they are using a product that works. In fact, as a whole, I would say that the major deficit to the product used was in the lack of essential information on the label pertaining to both product instructions and expectations.
Water test results over three week period:
| Day 1 | Day 2 | Day 3 | Day 4 | Day 6 | Day 7 | Day 14 | Day 16 | Day 21 | |
| FAC | 3.0 ppm | 7.5 ppm | 5.0 ppm | 3.0 ppm | 3.0 ppm | 1.5 ppm | 3.0 ppm | 3.0 ppm | 10 ppm |
| TAC | 3.0 ppm | 7.5 ppm | 5.0 ppm | 3.0 ppm | 3.0 ppm | 1.5 ppm | 3.0 ppm | 3.0 ppm | 10 ppm |
| pH | 7.7 | 7.7 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.6 |
| TA | 90 ppm | 110 ppm | 70 ppm | 70 ppm | 70 ppm | 90 ppm | 60 ppm | 40 ppm | 60 ppm |
| CH | 190 ppm | 190 ppm | 190 ppm | 190 ppm | 190 ppm | 190 ppm | 190 ppm | 190 ppm | 190ppm |
| CYA | 0 ppm | 0 ppm | 0 ppm | 0 ppm | 12:00 PM | 0 ppm | 0 ppm | 0 ppm | 0 ppm |
| Copper | 0.8 ppm | 0.4 ppm | 0.4 ppm | 0.4 ppm | 0.4 ppm | 0.25 ppm | 0.20 ppm | 0.20 ppm | 0.20 ppm |
| Temp | 86 F | 88 F | 86 F | 87 F | 88 F | 91 F | 89 F | 91 F | 91 F |
| TDS | 1,750ppm | 1,750ppm | 1,750 ppm | 1,750ppm | 1,750ppm | 1,750ppm | 1,750ppm | 1,750ppm | 1750ppm |
| LSI | -0.01 | 0.1 | -0.42 | -0.41 | -0.4 | -0.27 | -0.46 | -0.62 | -0.25 |
Kudos to Earth Science Laboratories who have generously offered to continue to supply the facility with AlgaeShield® algaecide so that the current swimming pool service company can continue the path of treatment until the current Cyanobacteria (Black Algae) problem is resolved. Sadly, there is still a long way to go. ?
To recapitulate our findings: a less than lethal dose of copper sulfate over an extended period of time will prove fatal to cyanobacteria in an actual swimming pool field test. It will take time, patience, and a copper test kit. We will need to monitor the copper level closely in order to both replenish that which is absorbed by the cyanobacteria and to stay beneath the EPA maximum level of 1.0 ppm in the EPA Safe Drinking Water Guidelines.
To see our research from May of 2018 that, with the phycology team at the University of Florida, had identified black algae as cyanobacteria click: the Black Algae Myth
Thank you to Thomas Race (Owner Aqua-Caribbean Swimming Pool Service, www.aqua-caribbean.com), for the use of the pool at his new account for chemistry experiments and for his assistance in this project.
Special thanks to Eli Ben-Shoshan ([email protected]) for meeting me early on a Sunday morning for the killer drone footage.
Histogram Results tallied by Grace Bennett
Huertas MJ, López-Maury L, Giner-Lamia J, Sánchez-Riego AM, Florencio FJ. Metals in Cyanobacteria: Analysis of the Copper, Nickel, Cobalt and Arsenic Homeostasis Mechanisms. Meeks JC, Haselkorn R, eds. Life. 2014;4(4):865-886. doi:10.3390/life4040865.
Amy Proal, Understanding Biofilms, Bacteriality, http://bacteriality.com






very interesting work. I am also testing some low-copper conc. methods in my home pool.
Nice! Keep us posted