[responsivevoice_button voice=”UK English Male” buttontext=”Listen to Article”]
What chemical lowers cyanuric acid in swimming pools and actually works? Believe it or not, it’s sold in the spice aisle of your supermarket and is an ingredient in Pickles.
Lower Cyanuric Acid Level, Video by Andrea Nannini of Hibiscus Pool & Spa.
Okay, so maybe I released this information before it has been deemed a proven method of reducing Cyanuric Acid levels chemically. This was a conscious decision, not something that I just blurted out on the spur of the moment. It’s not a statement that I wish I could retract. I have been experimenting with Aluminum Sulfate Al₂(SO₄)₃ as a means to Lower Cyanuric Acid Level (C₃H₃N₃O₃).
The Great CyA Debate in it’s Entirety Below ⬇
As I stated at the CyA debate at the IPSPE in New Orleans, I have had some success. I wanted to share that information because I do think it is something to get excited about. Maybe not bouncing off the walls with joy excitement, but a little enthusiasm toward the results.
Why announce it now?
To fully comprehend the reason, you must first understand that all of the research I conduct intends to make the industry better. I’m not looking to capitalize on my findings. I’m not funded, nor am I paid. All of our field tests to date have been 100% out of pocket and with the volunteer assistance of industry peers.
So why do it at all?
There are a lot of questions out there that we don’t have actual answers to. I mean, it’s 2019. Maybe there is something that was missed or not yet considered. Please take a look at the Black Algae research I conducted last year. I uncovered and had proven quite a bit of information that had been unknown up to that point. If we can come up with a low-cost means of lowering CyA without the need for RO or water replacement, this will certainly benefit the individuals that employ these methods.
To View, our findings on Black Algae, visit us at Black Algae Myth BUSTED.
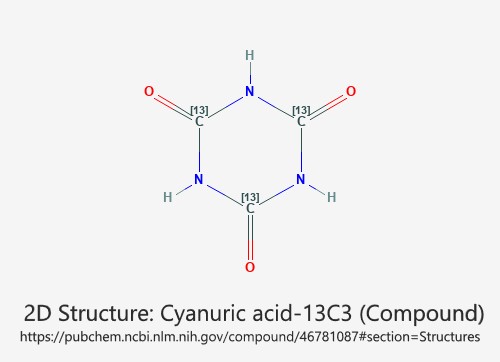
What made you think Aluminum Sulfate could be a solution?
I know adding melamine to the water will pull Cyanuric acid out of the solution, but melamine is a costly material. At a 1:1 ratio with CyA (it takes one pound of melamine to pull one pound of Cyanuric acid out of solution), implementing this procedure would be a costly venture.
I have used alum for many things over the years. Everything from removing Copper Stains from plaster to green to cleans on REO properties (foreclosed homes). Still, I had never thought of conducting a before and after the Cyanuric acid test until 2019, when a peer inquired about the possibility, noting a drop in ppm following a floc. This piqued my curiosity. I decided to perform a test and noted a dip in the level—nothing to write home about, but a drop nonetheless. Enthralled with the possibility, I spent the better part of the summer to see if I could create an alum-CyA removal method that would yield consistent and desirable results.
Lower Cyanuric Acid Levels.
Aluminum Sulfate Al₂(SO₄)₃ is the active ingredient in those two-pound cylindrical containers marketed as “Floc” that you see. The dose rate you typically find on those containers will only treat cloudy water at best. To utilize the chemical to its full potential, you’ll need to follow water treatment center guidelines at least as far as dose. Alum, those who have worked with the product, is also temperamental with particular pH and temperature requirements. The water needs to be at least 70°F or better. If the pH is too low, it will not work if the pH is too high, it will not work.
What’s the Best pH for Floc?
Even under normal circumstances, the question of the correct pH to use is controversial. Precedence and Water treatment facility use dictates a pH between 6.5 and 7.2 for best results. The instructions on those two-pound containers we spoke of a moment ago generally call for a pH of 8.0 or higher. The truth is, as you can see in the chart below, Alum will work well within a pH range of 5.8 to 8.0 with a bit of leeway on either end.
The level of aluminum sulfate in pickle juice is 110 ppm.
There is a difference, though. From other experiments I have conducted, I have found that it takes about twice the amount of alum to achieve a result at a pH of 8.0 than it does at 7.0
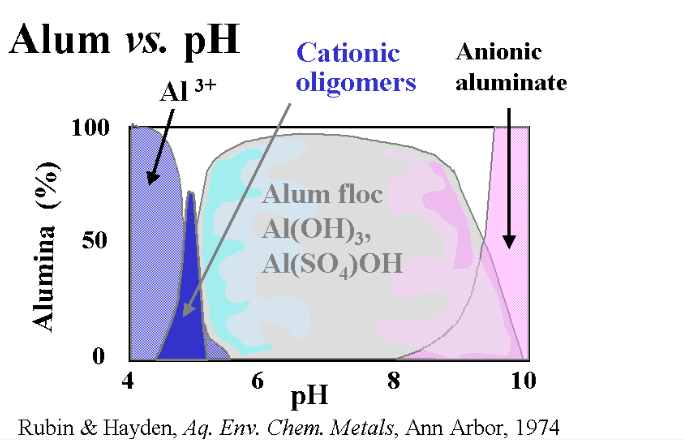
My initial thought noting the decrease in solubility of solids at a high pH, was to go high. I conducted my tests in a range of 7.8 up and through 9.0, well into the field where anionic aluminate becomes dominant. I did so with a water temp of 75°F and aluminum sulfate doses ranging from 25 ppm through 100 ppm. My results, at best, were minimal.
Lower Cyanuric Acid Levels.
Alum is Al2(SO4)3, when put in water it splits into Al+3 (aluminum) ions and SO42- (sulfate) ions. Water is H2O. It can split into H+ (hydrogen) ions and OH- (hydroxide) ions. The aluminum ions can combine with the hydroxide ions to form insoluble floc which is Al(OH)3. Since alum is taking out the hydroxide ions, we are left with a surplus of H+ ions. pH = -log[H+], so pH is a measure of the hydrogen ion concentration. When the number of hydrogen ions goes up, the pH goes down. When the number of hydrogen ions goes down, the pH goes up. That means that when you add alum and get a surplus of H+ ions, the pH will go down.
Back to the drawing board.
Okay, not the results I wanted, but I did have results. Besides, that was still somewhat exciting. I decided that I would go back to my usual MO for Alum and ride the pH low. I would repeat my experiment with my pH in a range between 6.0 and 7.2; the other parameters would remain the same.
Over 60% of the municipalities in the U.S. use aluminum sulfate for drinking water treatement.
Here is What Has Worked Best So Far.
Water Temp: 75°F Ideal (70°F – 90°F)
Alum Dose: 100ppm (8.33 lbs per 10K gallons) – this is the dose recommended for use in water treatment at municipalities.
pH: 7.0
All other Values (TA, CH, TDS) were within the recommended ranges. Total Alkalinity at 90 ppm
Water temp >70F
With that, I have achieved a reduction of Cyanuric Acid from starting levels of 90 to 100 ppm by 50%. There is still much more testing to be done, and at this point, I am uncertain if I had achieved a 50% drop or merely a 50ppm drop due to my start point. The time required to make the noted change was twelve to fourteen hours. Circulate bypassing media following dose then stagnant twelve hours before an extremely slow vac to waste.
Though positive results have been achieved, this experiment is still in its infancy and is far from being considered as proven. Honestly, I think it needs to be successful hundreds, if not thousands of times before; I would say it’s a success. This is where feedback from others, before and after CyA levels, that floc with alum in high doses would be beneficial.
I know the dose rate of 8.33 pounds per 10,000 gallons sounds expensive, and if you are purchasing those two-pound bottles of “Floc,” it will be. Alum is available for water treatment centers in fifty-pound bags and at the cost of somewhere between $25 and $35 each and can be purchased through swimming pool distribution centers.
Alum is commonly used in hospitals as a blood coagulant in wound care.
Do Not Try This At Home!
This is clearly only a process that should be attempted by a Swimming Pool Professional. You’ll need to be well versed in the EXACT method of adding a dose of alum to include filter run time, attention to pH level, precautionary measures for filter equipment. You’ll need to be prepared to slowly vacuum a massive amount of “sludge” from the pool floor with a portable vac system (Not a Hammerhead or Riptide style vac).
Please keep in mind there is still much tweaking (not twerking, that’s Cyrus, not CyA) to be done. If you are a Pool Professional and utilize alum in a high dose, I would greatly appreciate it if you shared your before and after CyA results. This is how we go from my statement of “some success” to a proven method. Please let us know in the comments below.
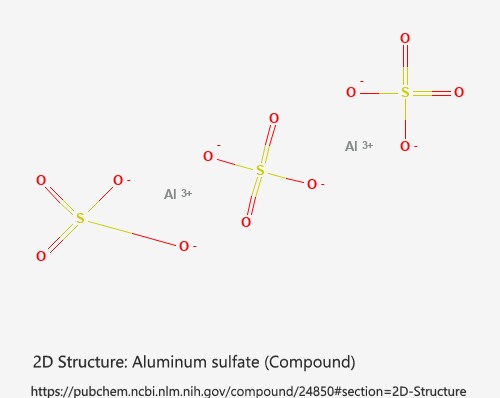
Here’s why this would be great.
Alum is extremely inexpensive and easily obtainable. It may take some convincing to get your distributor to supply product in a bulk size, but it is definitely available to them. Even on the high end at, let’s say, $35 per fifty-pound bag, that works out to $0.70 per pound. At a dose of 8.33 pounds per 10K gallons, the total cost of chemical (outside pH adjusters) would be $5.83
A thought on why this is working.
We are aware that aluminum sulfate will remove dissolved phosphates from solution, so it is common knowledge that it is not a product that is limited to removing suspended solids. We are also aware that Cyanuric acid will readily form complexes with metals; this is evidenced in copper-cyanurate, where a soluble CyA forms an insoluble compound. So I theorize the formation of an aluminum-cyanurate complex Al(H2C3N3O3)3 and precipitation of such expedited by the formation of aluminum hydroxide (Floc). Of course, this is all purely conjecture.
NOTE: Aluminum sulfate or any sulfate product should not be used in a saltwater pool at high levels. It may shorten the salt cell (Manufacturers do not provide information on concentration). High sulfate levels (> 300 ppm) can cause damage to stonework. Aluminum sulfate should not be used in a pool with pigmented (colored) plaster, as some have reported bleaching to occur. A swimming pool should be tested for residual aluminum following use, and EPA Secondary Contaminant Aluminum max level of < 0.2 ppm maintained.
Update – 10/23/2020: The members of the Talking Pools FB group have been field testing this method for greater than 8 months at this point with identical positive results. None have noted an increase in CyA following the initial removal in periods as great as six months following.
Update – 11/04/2020: Chemist Marcelle Dibrell (Service Industry News) located a published research document that confirms my theory that Aluminum (supplied by our aluminum sulfate) will form insoluble aluminum-cyanurate complexes: AlC3N3O3 · 3H2O – Seifer, G.B. Cyanuric Acid and Cyanurates. Russian Journal of Coordination Chemistry 28, 301–324 (2002). https://doi.org/10.1023/A:1015531315785
Update – March 31,2024: onBalance, a swimming pool industry consulting and research firm, confirms with lab results that Alum CyA removal method will successfully lower Cyanuric Acid levels in a swimming pool. See Service Industry News Announcement






I personally have had almost a 50% drop on cya by just adding 8 lbs per 10k of water in a little over 12 hours on a 19000 gallon pool with a water temp of 80 degrees.
Perfect! it’s that 8 lb per 10K gal dose. awesome.
Pool #1 Saturday June 13
19000 gallons
Cya was over 100 did not get an exact number but end number after a single alum treatment of 8.3lbs per 10k of water was at 70
Pool #2 Saturday June 13
12000 gallons
Cya was over 100 did not get an exact number but end number after a single alum treatment 8.3lbs per 10k of water was 80
This pool was also a green to clean
Here’s our test results
13565 gallons
start date 6/15/2020
hardness 1000+
tc 5
fc 5
pH 7.0
TA 120
Temp 79.75
TDS 3626
CYA 630 !! measured @ 6:1 dilution with pentair kit
added 10.4 lbs Alum, recirc 2 hours, all equipment off for 48hrs
6/17/2020
Hardness 1000+
tc 3
fc 3
pH 6.2-6.4
TA 80
Temp 80.9
TDS 3660
CYA 315!! measured @ 6:1 dilution with pentair kit
From a Cyanuric Acid level of 630 ppm to 315 ppm in two days without draining. Phenomenal!
7/27/2020 Very dark green pool
8lbs Alum added per 10,000 gal
CYA: 80
pH: 7.8
Phosphates: 100
7/29/2020 Vacuumed bottom to waste
CYA: 55
pH: 7.0
Phosphates: 0
Result: Clear pool, lower pH, lower alkalinity, lower CYA, lower phosphates.
Water depth 4 ft, average water loss 4 in, testing using Taylor 2006 kit, sample taken with water at high level after recirculation for 1 hour at 18-in depth, second sample taken 24-48 hrs later after vacuum to waste but before additional water added.
7-25-2020 though 7-26-2020
Pool #1
Cl-8
Fc-8
Ph7.2
Ta-100
Ca-400
Cya-700
Day-2
Cl-8
Fc-8
Tds700
Ph of approximately-6.8
Ta-60
Ca-400
Cya-100
Tds-690
This was done with alum at a rate of 8lbs per 10k of water
7-25-2020 though 7-26-2020
Pool #2
Cl-10
Fc-10
Ph-7.4
Ta-70
Ca-280
Cya-100
Tds390
Day -2
Cl-10
Fc-10
Ph of approximately 6.6
Ta-30
Ca-280
Cya-80
Tds-390
This was done by using alum at a rate of 8lbs per 10k of water
No water was added it was over full on day 1
8-1-2020 though 8-2-2020
Day 1
Cl-8
Fl-8
Ph-7.6
Ta-100
Ca-380
Cya-140
Tds-1100
Day2
Cl-8
Fc-8
Ph of approximately 6.6
Ta-80
Ca-360
Cya-90
Tds-1100
This was done with using alum at a rate of 8lbs per 10k of water and no added water due to it was already over full
Well these are fantastic results.
Personally I avoid Aluminum sulphate or floc if I can, choosing DE to get rid of red rain…. But, this sounds like a great plan.
I am thinking of this as a spring program to adjust badly managed pools, as well as an emergency procedure.
I also think pressure should be applied to pool shops in particular, to sell tablets and granules in the same way as cigarettes I. E these products will seriously damage your pool…. On the basis of cause and effect.
I also think more effort is required to provide peristaltic pump technology to dispense liquid chlorine and sulphuric acid as “basic” level maintenance. This therefore avoiding trichlor over stabilisation in the first place….
Back to aluminium sulphate, sounds like a plan, and as you say one that needs careful handling….
Crickey 600ppm CYA levels. That suggests the pools are clean only because of copper sulphate (algacida in trichlor mix) that is horrible!!!!
Pingback: Cyanuric acid shortage looming - Pool Operator Talk News🗞