If you’ve been following along, I’m sure you’ve noticed that I’ve been spending a lot of time talking about specialty chemicals lately. That’s because the availability of the critical disinfectants we have always used has become highly unreliable. And, with the predicted cyanuric acid shortage – there is no light at the end of the tunnel. So, today I wanted to talk a little about how enzymes work.
We are at a stage where we find ourselves needing to add products to our protocol of care that we maybe hadn’t used much in the past. These will not enable us to go without chlorine completely, but adding specialty chems on the reg will make it, so your pools don’t need anywhere near as much chlorine as they did in the past.
How enzymes work
Before we jump into this, I need you to have a clear mind. I need you for just a minute to forget everything everyone has ever told you about enzymes and how they work. That doesn’t mean I’m saying it’s all bullshit like many things; it’s more like facts with a sprinkling of bullshit—first things first -don’t ever let anyone tell you that enzymes digest anything. That’s plain and straightforward shmegegge. Enzymes cannot digest. They are not alive, and digestion is a function of living organisms.
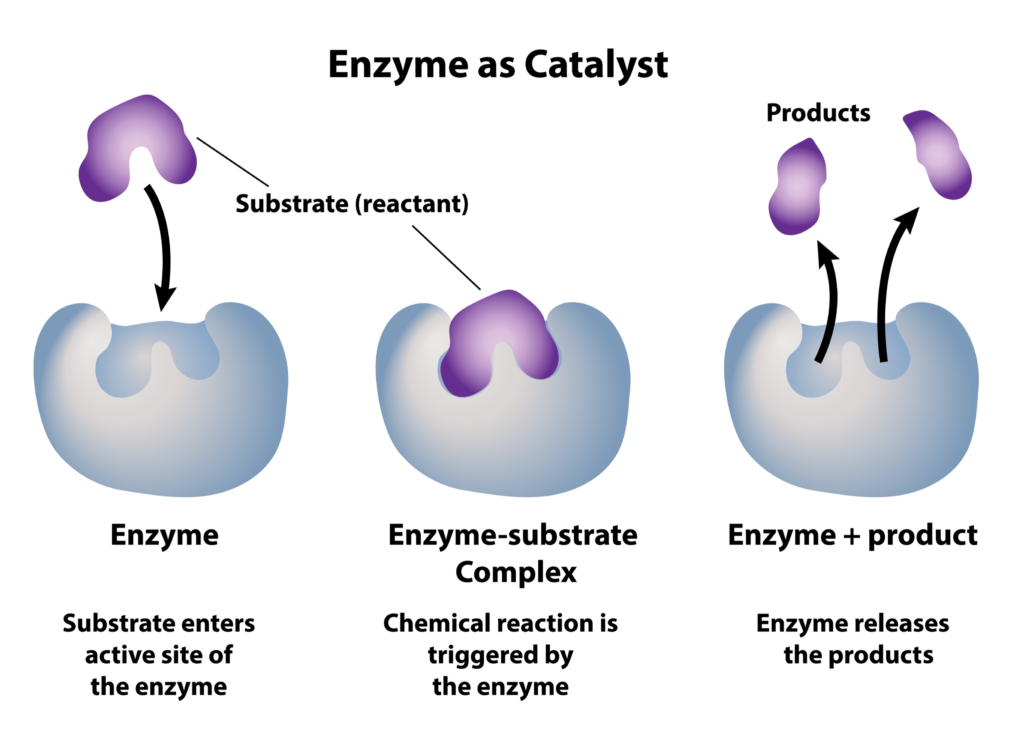
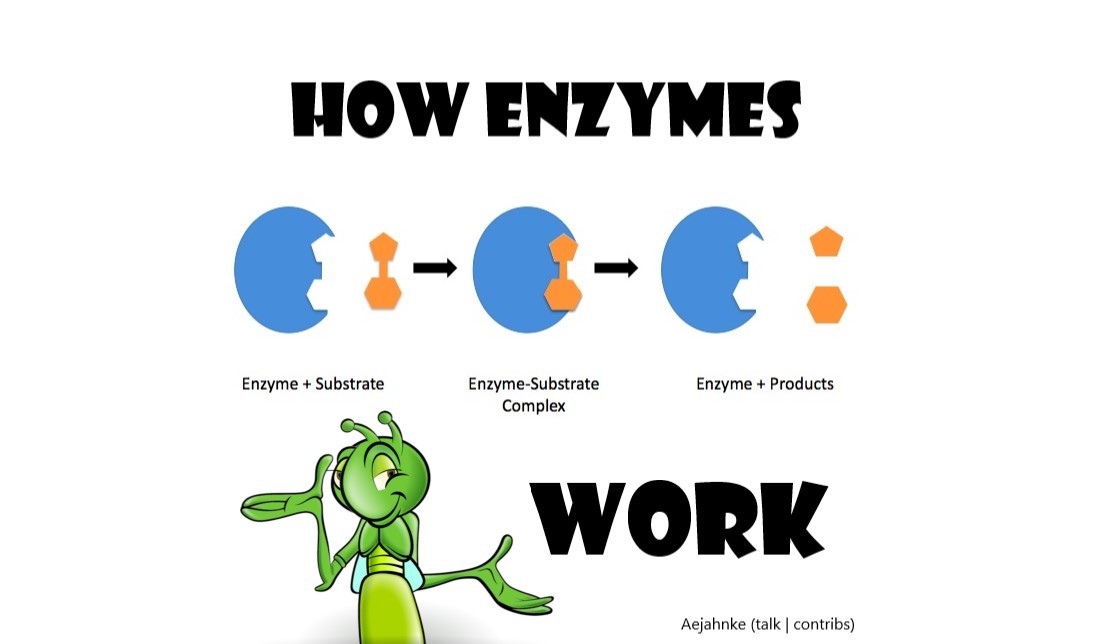
What enzymes actually do is catalyze oxidation reactions of organic compounds (substrate) so that bacteria in the water can eat the schmutz (products). The bacteria then digest those products converting them to CO2 and H2O. So this oxidation reaction gives us something that bacteria can more easily consume – A less complex product. This is why bio-enzymatic products generally work best.
The specific action of an enzyme with a single substrate can be explained using a Lock and Key analogy first postulated in 1894 by Emil Fischer. In this analogy, the lock is the enzyme and the key is the substrate. Only the correctly sized key (substrate) fits into the key hole (active site) of the lock (enzyme).
June 5, 2021, What is the lock and key theory of enzymes?, Rehabilitation Robotics
^^^ This explains how enzyme concoctions can be formulated to work on a specific substrate (organic compound) type.
Harvested from bacteria
Try to remember there are both good and bad bacteria. Also, whether the product is bio-enzymatic or not, bacteria were involved at some point in time. You are using either a liquid with a combo of living bacteria (the good kind) with enzymes in it, or you are using enzymes alone that were extracted from bacteria.
Enzymes flying solo do still serve a purpose when void of microbes (bacteria). They do still help to catalyze these reactions where carbon atoms gain bonds to oxygen. If four oxygen molecules bond to a carbon atom, it is oxidized to CO2. Similar to Khaleesi, enzymes are the ‘breaker of carbon bonds’ #GOT. However, carbon bonds to carbon are kick-ass strong, and if a compound has two or more carbon atoms, this compound cannot oxidize to the point that it bubbles off as CO2 (carbon dioxide).
Enzymes catalyze oxidation reactions
In most cases, after the carbon atom has been oxidized by microbial oxygenases (one of three enzyme groups used in water treatment), the dissolvability of the products (from the substrate) increases and ultimately contributes to TDS (Total Dissolved Solids). Or, in the case of swimming pools, HOCl (hypochlorous acid) will aid in ridding the pool of that which doesn’t dissolve.
Another popular group of enzymes used in water treatment is microbial laccases (p-diphenol: dioxygen oxidoreductase). Different types of bugs and fungus create these copper-based enzymes. Oxidase (the enzyme type here) catalyzes an oxidation-reduction reaction and in this reaction, the result is hydrogen peroxide.
Some brands of enzymes for swimming pools contain alcohols, C9-11, ethoxylated as the active ingredient. This is simply a combination of emulsifiers and surfactants. The emulsifier causes the oil-type gak that sheen” across the surface to form droplets where the surfactant reduces the surface tension allowing it to sink a bit. The process makes removing the contaminant during filtration easier. This chemical is not subject to the same chlorine interference (oxidation) as the concoctions above.
Heavy metals in the formula
Other enzyme products boast phosphate removal on top of the enzyme function. If this is the case, it is because that chemical contains heavy metals in the enzyme product. Lanthanum is the go-to metal used for phosphate removal.
I strongly recommend adding specialty chemicals to your routine
Rudy Stankowitz
No matter which specialty chemical you purchase or what brand you use, always follow label instructions for optimum results. Keep in mind that a chlorine level in the pool can oxidize the enzymes you are adding. Always obtain a copy of the product SDS. I always check the ingredients – personally, I won’t add a product if I don’t know what’s in it. Wear the PPE (personal protective equipment) recommended. Trust me, after COVID-19; no one is even going to notice you have it on.
.
ref:
Simple Science of How Enzymes Clean – Enzymes and Bacteria Together (nycoproducts.com)
17_redox_states_carbon.pdf (utdallas.edu)
Top 3 Ways To Use Enzymes In Wastewater Treatment (infinitabiotech.com)






Pingback: How Phosphate Removers work - Pool Operator Talk News🗞