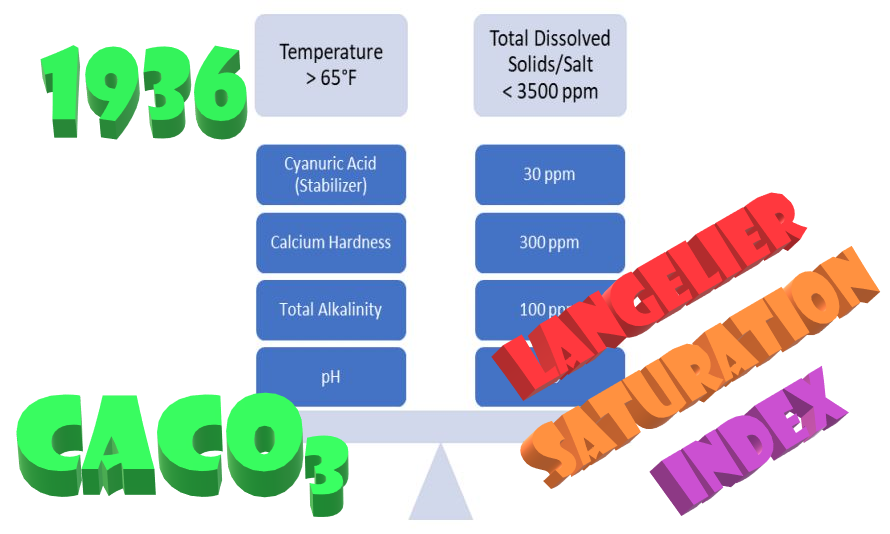
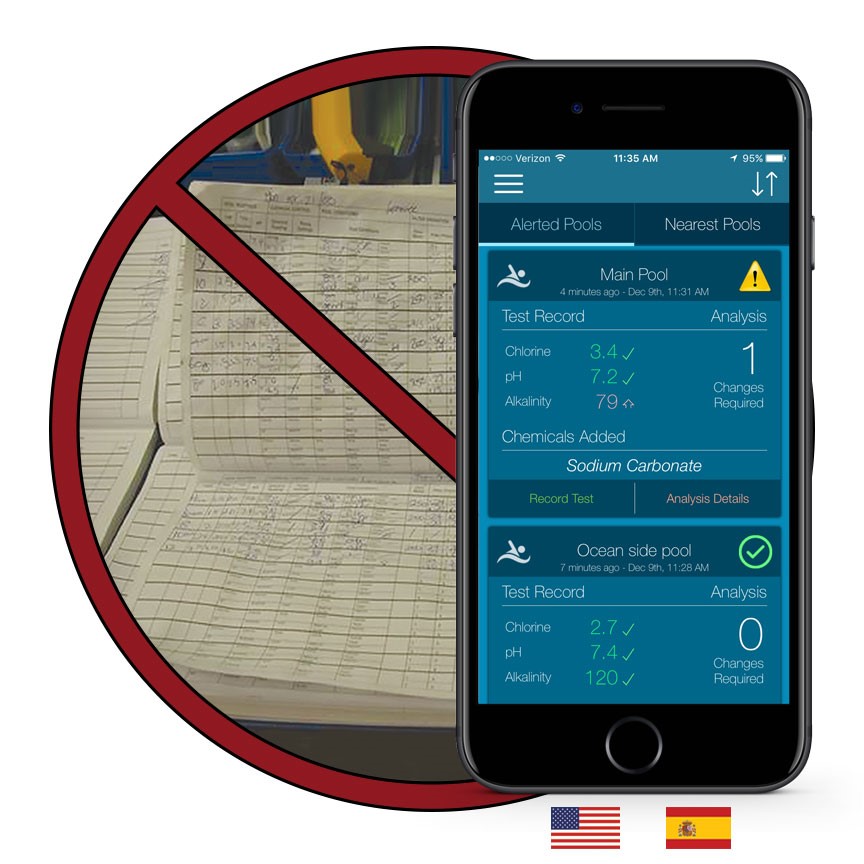
Wilfred Langelier’s formula
Wilfred Langelier's formula for predicting calcium carbonate precipitation potential (CCPP) is a widely-used method for assessing the scaling potential of water. Langelier's formula, also known as pHs, measures the tendency…