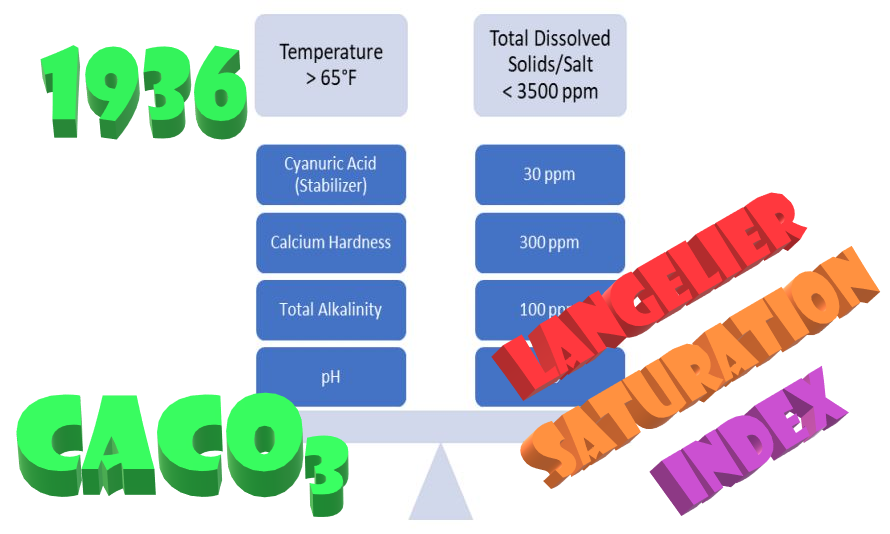
The Langelier Saturation Index is the basis of swimming pool water chemistry. A simple mathematical formula determines whether the water is in balance (neither corrosive nor scale forming). Realistically, not a measure of the success in preventing algae or bacteria, but a calculation designed specifically for the protection of the vessel, the swimming pool itself—a determination of calcium carbonate saturation. Let’s face it; we have come to depend upon the accuracy of the pool industry LSI (Langelier Saturation Index).
The water being in balance is extremely important, and the LSI is a tool we rely upon. After all, we know water needs to have a certain amount of calcium and carbonate in solution, and the level we need varies upon both temperature and pH. If the water is found to be corrosive, it will pull calcium and carbonate from the pool walls and floor, etching the surface. When the water is scale forming, everything from a cloudy water condition to calcium carbonate deposits forming about the pool.
This Photo by Unknown Author is licensed under CC BY
Many think calcium carbonate saturation is only important in plaster pools, and that’s not the case. Nowadays, liner companies utilize calcium carbonate in the production of vinyl. The amount used varies from manufacturer to manufacturer, but it has been found that corrosive water can pull calcium carbonate from vinyl when the content is greater than 7%. It is also a fact that cobalt staining is more prevalent in fiberglass pools where a corrosive saturation index persists.
Dr. Wilfred Langelier, professor emeritus of civil engineering at the University of California, Berkeley published this concept in a paper titled The analytical control of anti-corrosion water treatment in 1936 in the detailing the model was first published in 1936 in the Journal of the American Water Works Association. Most are aware of this; however, a common misconception is that we actually use Dr. Langelier’s model, which was designed to determine the Potential for calcium carbonate precipitation. In actuality, we do not.
A Mother of a Math Problem
Langelier’s formula, despite its accuracy, is extremely complex. It also was not designed to determine whether water was corrosive or scale forming. Instead, as I mentioned above, the calculation only determined the calcium carbonate precipitation potential (CCPP). Not a guarantee that a problem with calcium would occur, only a predictive measure of an increased possibility. Regardless, Langelier (already widely known for his advances in water treatment due to studies documented in his 1921 paper Coagulation of Water with Alum by Prolonged Agitation) came up with a highly complex formula. That was beyond practical for longhand calculation – the math was insane!
View the entire Journal of the American Water Works Association 1936-10, Vol 28 HERE
Due to its complexity and advances in science, the formula was simplified in 1965 by Carrier (heating, air-conditioning, and refrigeration solutions). This new version is often referred to as the ‘improved’ version and was the first to call the formula the Langelier Saturation Index. Oh yeah, I almost forgot – pHs is the name Langelier gave to his formula for predicting calcium carbonate precipitation potential.
Is Carrier’s LSI improved? Answering that question is beyond my skill set, but I can say it definitely dumbed it down. With that, of course, some suggest this revised 1965 method is too simplistic to be as accurate. However, this new calculation is more similar to the formula that we use now in the swimming pool industry. That’s right; we’re not up to what we use for swimming pools just yet. That doesn’t happen until 1974.
Change is good, right?
This was when John A. Wojtowicz of Chemcon got his hands on it. Wojtowicz pointed out that the LSI in its current version did not consider other alkaline substances that would contribute to the Total Alkalinity in a swimming pool (i.e., cyanuric acid, boric acid, etc.). So, the formula was retooled once again. See The Effect of Cyanuric Acid and
Other Interferences on Carbonate Alkalinity Measurement in the Journal of the Swimming Pool and Spa Industry
This is where we are at now – the Wojtowicz Saturation Index? Not sure if this name will stick, but it is certainly more accurate than calling is LSI. Misnomer or not, this is the saturation index calculation that we use in the pool industry today. This is where the formula for the LSI apps we use originated. Accuracy in attribution or not, is it more accurate in predicting the potential for calcium carbonate to precipitate in an open body of water?
I thought it would be interesting to see the difference in calculation between the three using values we would likely see in a swimming pool, specifically omitting the presence of cyanuric acid, borate, or any other substances that might affect our Total Alkalinity.
Our example water test:
Water Temperature
pH
Calcium Hardness
Total Alkalinity
Cyanuric acid
Borate
Phosphates
Total Dissolved Solids
81° F
7.5
300 ppm
90 ppm
0 ppm
0 ppm
0 ppm
1,000 ppm
Saturation Results:
Version
1936 – Dr. Wilfred Langelier – pHs
1965 – Carrier – LSI
1974 – Wojtowicz – Pool Industry LSI (using a currently popular industry app)
Index Calculation
7.3
.
0.24
.
0.00
Determination
Supersaturated – Scaling possible
Scale forming and corrosive
In Balance – not scale forming, not corrosive.
A Formula never intended to be used in an open body of water exposed to atmospheric pressure 🤷♂️
Another factor to consider – The saturation index is said to only be accurate within certain parameters. Luckily, the chemistry we keep in swimming pools typically falls within that range. That is except for Saltwater pools.
- pH: 6.5 to 9.5
- Temperature: 32 to 212°F
- Bicarbonate alkalinity: 10 to 800 ppm
- Calcium hardness: 50 to 700 ppm (up to 900 ppm)
- Total dissolved solids: 50 to 1000
Accuracy of the pool industry LSI
If Dr. Langelier’s pHs is the more accurate measure of determining the water’s potential of calcium carbonate precipitation and was truly only changed for ease of calculation, with the capabilities of cell phones, wouldn’t it make more sense to develop an app that could quickly perform the complex equation? Of course, Wojtowicz’s Carbonate Alkalinity would be a necessary modification in a swimming pool application.
If not, and today’s LSI method continues to be the route we take, do we really need an app or studies on mathematical calculations at all? I mean, the reality of it is shooting for the dead center of the ideal range on the three values for which an ideal range exists; the water will always be in LSI balance down to a temperature of 48°F and as high as 104°F anyway. The exception being a saltwater pool in which none of the calculations are apparently accurate anyway.
Specific Parameters
Even if the inaccuracy of the LSI at a Total Dissolved Solids (TDS) level above 1,000 ppm did not exist, maintaining a pH of 7.5, Carbonate Alkalinity of 90 ppm, and a Calcium Hardness of 300 ppm would keep the water balanced on today’s pool industry LSI balanced in water temp range of 63°F to well over 104 degrees (TDS of 3,000 ppm).
Have an LSI app on your phone? Try it! Set the pH at 7.5, Carbonate Alkalinity at 90 ppm, and the Calcium Hardness at 300 ppm. Now play around with different numbers for temperature and TDS. Do we really need an app? Or, would a simple dosing calculator suffice?