Cyanuric acid is a white, crystalline powder commonly used in the swimming pool industry as a stabilizer. It helps slow chlorine decomposition in sunlight, reducing the amount of chlorine needed to maintain the target chlorine level in a swimming pool. Cyanuric acid is also used in swimming pools in trichloro-s-triazinetrione (trichlor), stabilized chlorine.
Heating up some urea
Cyanuric acid is made by the thermal decomposition of urea. Urea is an odorless solid that is shed in urine and is also used in fertilizers and plastics. The thermal decomposition of urea is a two-step process; urea is heated to a temperature of about 175 degrees Celsius. This causes the urea to break down into cyanuric acid and ammonia. In the second step, the ammonia is released from the cyanuric acid. This is done by heating the cyanuric acid to a higher temperature, about 250 degrees Celsius.
The overall reaction for the thermal decomposition of urea to cyanuric acid is:
3 H2N-CO-NH2 → [C(O)NH]3 + 3 NH3
The cyanuric acid that is produced is typically a mixture of the dihydrate and the anhydrous cyanuric acid, which is more stable, and is the form that is usually used in swimming pools and spas.
www.chlorine.org
The Trichlor Process
The first step in the manufacturing process of trichloro-s-triazinetrione is to create a solution of cyanuric acid and sodium hydroxide. This solution is then heated to a high temperature to produce cyanuric acid anhydride. The reaction occurs in a reactor vessel that handles high pressures and temperatures.
similar article Cyanuric Acid, Chlorine Lock, and the CDC
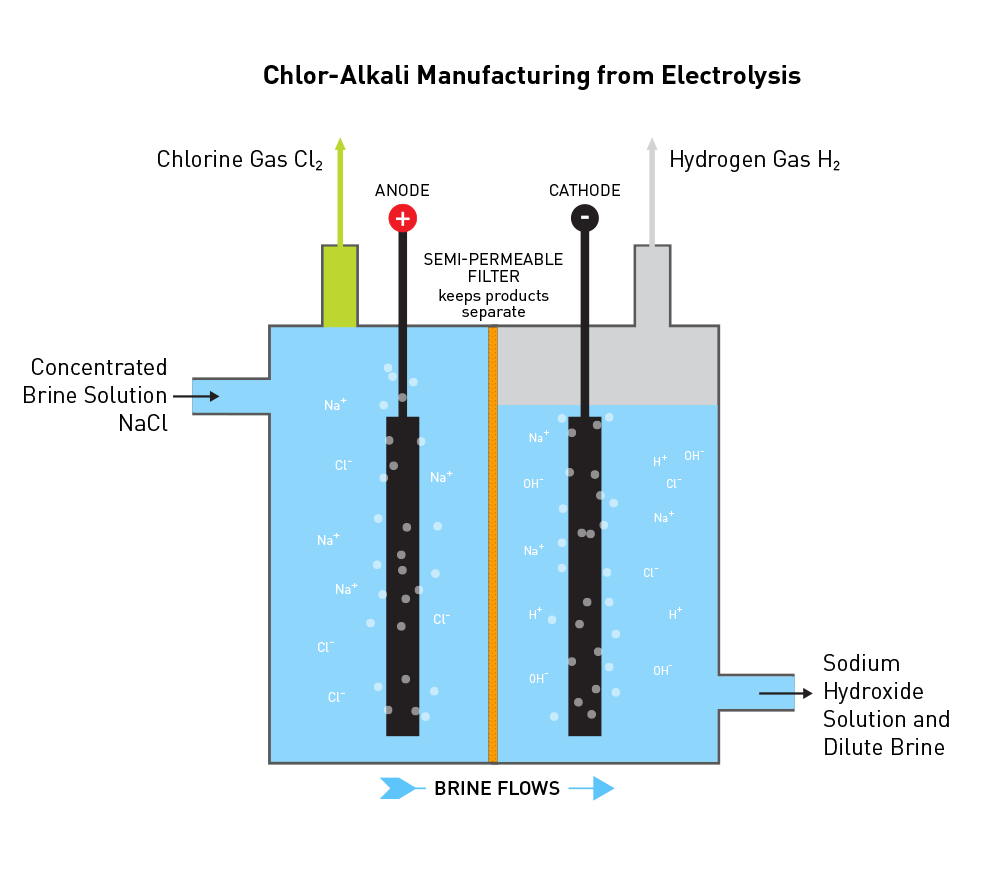
Chlorine is manufactured in a process called Chloralkali. This involves running an electric current through a salt (brine) solution, which gives you chlorine. Alongside chlorine, you also get other helpful stuff like caustic soda (sodium hydroxide, NaOH) and hydrogen gas (H2).
A cyanuric acid anhydride is then reacted with chlorine gas in the presence of a catalyst, usually iron or aluminum, to produce trichlor. The reaction occurs in a separate reactor vessel that handles high pressures and temperatures.
It’s getting hot in here
This chlorination reaction releases a significant amount of heat. Trisodium salt is typically produced by reacting cyanuric acid with sodium hydroxide in a specific ratio, usually around 1.0 to 3.0 moles. Similarly, Dichloroisocyanuric acid (Dichloro-S-Triazinetrione) is formed by chlorinating the disodium salt of cyanuric acid. To create the disodium salt, cyanuric acid is reacted with sodium hydroxide in a ratio of approximately 1.0 moles of cyanuric acid to 2.0 moles of sodium hydroxide.
similar article Chlorine Facts
The same equipment is commonly used for the industrial production of Dichlor (Dichloro-S-Triazinetrione) and trichlor. This is because the chemical reactions in producing both Dichlor and Trichlor utilize the same essential substances in different proportions. However, switching between the production of Dichlor and Trichlor requires the process to be temporarily stopped or adjusted, which comes with its own challenges.
Have it your way
Once the reaction is complete, the trichloro-s-triazinetrione is removed from the reactor vessel and cooled. Depending on the manufacturer’s preference, it is processed into the final product as a tablet or granule form.





